New Delhi, Jul 27: The Supreme Court on Thursday granted an extension of tenure to Enforcement Directorate chief Sanjay Kumar Mishra till September 15 but made it clear there will be no further extension.
The Centre was seeking an extension in Mishra's tenure till October 15.
A bench of Justices B R Gavai, Vikram Nath and Sanjay Karol said it was granting the extension in "larger public and national interest" but that Mishra will cease to remain ED chief from the midnight of September 15.
During the hearing, the top court questioned the Centre for seeking an extension and asked if the entire department is "full of incompetent people" except the incumbent chief.
"Are we not giving a picture that there is no other person and the entire department is full of incompetent people?" the bench told Solicitor General Tushar Mehta, appearing for the Centre.
The top law officer argued that the continuity of the ED leadership is necessary in view of the Financial Action Task Force (FATF) peer review whose rating matters.
Mehta said Mishra is "not indispensable" but his presence is necessary for the entire peer review exercise.
Representing the ED, Additional Solicitor General S V Raju said, "Some neighbouring countries want India to fall into FATF's 'grey list' and therefore, the ED chief's continuity is necessary".
The bench was hearing the Centre's application seeking the continuance of Mishra's tenure till October 15.
The top court had on July 11 held as "illegal" two successive one-year extensions granted to Mishra and said the Centre's orders were in the "breach" of its mandamus in the 2021 verdict that the IRS officer should not be given further term.
It had also curtailed Mishra's extended tenure to July 31 from November.
Let the Truth be known. If you read VB and like VB, please be a VB Supporter and Help us deliver the Truth to one and all.
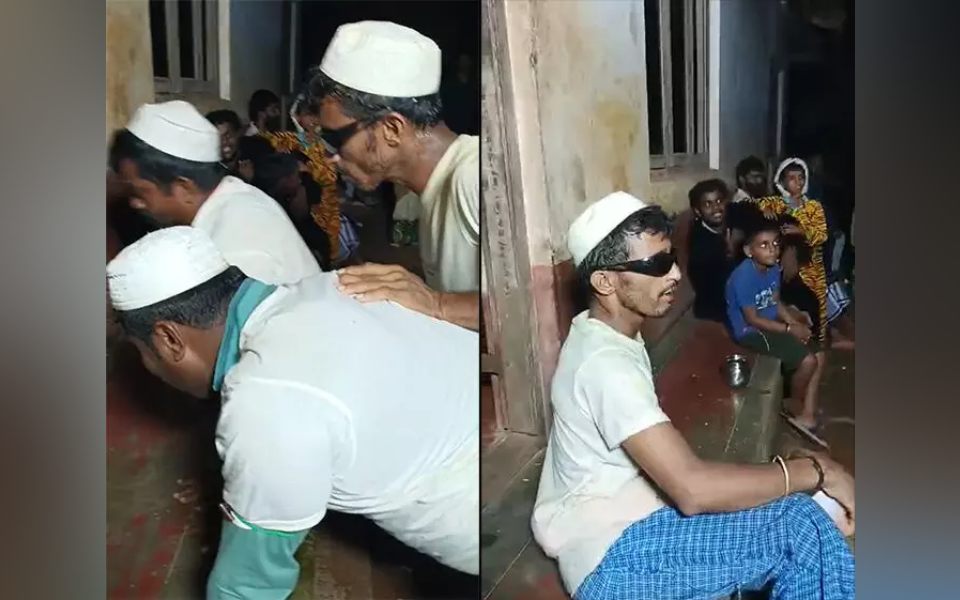
Belthangady: Another incident involving individuals allegedly dancing indecently, dressed in a denigrating manner, and insulting the Azan (Islamic call to prayer) has come to light under the jurisdiction of the Venoor Police Station. A case has been lodged against 17 accused persons and 20 to 30 others who were allegedly involved in the act.
Based on a complaint filed by Mohammed Rafiq, a resident of Poyyegudde house in Padangady village, a case has been registered at Venoor police station against Savya residents Vasantha, Rajesh, Harish, Peradi residents Dayanand, Ranganath, Mohan, Shamit, Ravi, Ramesh Kulal, Suresh, Ashoka Kulal, Dhanush Haradottu, Harish Banthottu, Ramesh R.K, Suresh Barottu, Pramod, Prakash Peradi and 20 to 30 other individuals.
A case has been registered under Section 353(2) along with Section 3(5) Bharatiya Nyaya Sanhita 2023 for disrespecting Islam, Prophet Muhammad and the Islamic prayer Azhan by wearing Muslim attire during a 'Purusha Kattuna' event on April 14th.
A video of the incident is reportedly circulating on various social media platforms. In the purported video, a group of individuals can be seen wearing traditional Muslim attire and allegedly insulting the Azan by dancing indecently during a 'Purusha Kattuna' (a ritualistic dance performed by men of different castes, typically marking the end of agricultural activities) event held in Peradi near Venoor.
This incident follows a similar episode reported just days ago during a Purushara Puja ceremony, where Islam was allegedly disrespected. A complaint had already been filed with the Venoor police regarding that incident.
Leaders from the Social Democratic Party of India (SDPI) had also approached the Venoor police and submitted an official complaint, and demanded immediate action against those who are responsible for creating unrest in the society by wearing Muslim attire and hurting religious sentiments.
The Venoor police have now registered a case following widespread outrage over the alleged video denigrating Islam.