New Delhi: A few weeks ago, AstraZeneca, the company that developed the ChAdOx1-S/nCoV-19 [recombinant] vaccine, 175 crore doses of which were given in India under the name Covishield, admitted in a London court that its vaccine could have caused blood clots, heart attacks, and strokes, and later, on May 5, 2024, said it was withdrawing that vaccine. People who have lost their loved ones due to side effects or who have suffered serious or permanent disabilities are filing lawsuits against the makers of COVID vaccine companies in many parts of the world seeking compensation. Such cases have also been filed in India in the past and the statement by AstraZeneca has added new momentum to these complaints.
As the company acknowledged the serious consequences of the vaccine, the anxiety, suspicion and anger of those who had received the vaccine have increased further. While the government that gave the vaccine and the company that produced and sold it have remained silent, the doctors who supported and encouraged the vaccination are now struggling to offer justifications.
Was COVID-19 the worst, deadliest infection ever? Could the highly contagious corona virus have been prevented by a vaccine? Was it appropriate to vaccinate while the Corona infection was rapidly spreading? Was it necessary to vaccinate even those who had already been infected with the new Coronavirus? Was it necessary to vaccinate young and healthy adults? Was there clear evidence about the safety of new corona vaccines?
If the answer to all these questions is ‘yes’ then it was right to give the vaccine, if the answer is ‘no’ then the vaccine should not have been given.
Was COVID-19 the worst, deadliest disease? No, and that was known in January 2020 itself, within two months of the spread of SARS CoV2 virus. It was by then estimated that the risk of death from the new corona infection could be 3 to 20 per million among those under the age of 50, and more than 60 per million for those over 50 (this estimate was so accurate that deaths from COVID in 38 countries before the vaccines were introduced were less than a third of this initial estimate!). Only on the basis of such estimates some of us had argued that young adults did not need any vaccine at all as the chances of serious problems from corona infection were expected to be very low in that age group. But ignoring all the evidence, everyone from children to the elderly was given the vaccine. Covishield and Covaxin were given to those above adolescence, and Corbevax vaccine, which was not used elsewhere, was given emergency use authorisation for vaccinating younger children.
Instead of answering whether a vaccine could prevent the transmission of coronavirus, which easily spreads from one to six people through inhalation, many new narratives were created. Initial reports claimed that the Corona vaccines are 78 percent effective against symptomatic infection, 86 percent against hospital and ICU admissions, and 87-100 percent against death. But as days rolled by, these positions changed, and it was stated that the vaccines cannot be guaranteed to prevent infection, and more new vaccines may be needed as new variants evolve. In the same breath, it was also stated On the back of that, the vaccine will prevent the spread of corona, it was continued to be said that everyone should be vaccinated, and this was escalated further to the extent that the media and else started campaigning that those who were not vaccinated would endanger the entire community, children who could not be vaccinated would be a danger to teachers, so schools should be closed, those who were not vaccinated should not travel anywhere, should not be allowed to enter anywhere, not even to go to playgrounds, schools, or even hospitals, not work as nurses, in general, it was portrayed as though the unvaccinated were sociopaths, had no right to live, and were ostracised, fired from jobs, even arrested. In India, within days of the vaccination drive for the public from April 2021, instead of decreasing, the spread of Corona started to rise again, hitting as the second wave, proving the futility of vaccination as a measure to control transmission of SARS CoV2.
Was it appropriate to vaccinate while the Corona epidemic was spreading rapidly? It was for the first time in human history that a vaccine for a highly contagious, minimally harmful coronavirus was developed in haste, and given to everyone while the infection was spreading, even though it was not guaranteed to prevent infection. It is not easy to understand the logic behind the grand plan to vaccinate hundreds of crores of people with two doses of vaccine to prevent an infection that spreads easily to one to six people from an infected person! Whatever, the virus infected everyone much faster than the vaccine, and even for the already infected individuals, the vaccine was also injected!
It is common knowledge that infection with any virus, including Corona, confers lifelong immunity. In India, by December 2020 the central government had said that 40 percent of the people had been infected with Corona, and the Hon'ble Prime Minister had announced on January 18, 2021 that India had successfully won the war against Corona. If that was indeed the case, we asked, what was the need to vaccinate 40-60% of Indians who had developed immunity against infection? In natural infection, with the body being exposed to innumerable viral particles, strong immunity is produced against all 29 proteins of the virus, and antibodies are developed against not only its spike protein, but also against other proteins, whereas, following vaccination, our body gets exposed to a limited amount of selected viral particles, spike protein alone in the case of SARS CoV2, and develops limited immunity, that too unproven. Despite these facts, a new argument was floated all over, without any evidence, that the new Corona vaccines would provide stronger protection than the natural infection! Although it was clearly established that the vaccine did not prevent infection or provide better and longer immunity from the original strain or variants, or re-infection, even those who had recovered from the infection were pushed into vaccination even though it was clearly established that those already infected had good, long-term immunity and the chance of re-infection was very small.
By the time the Corona vaccine was available, a year's experience with Corona infection was available globally, including in India. By then it was clear that there were no complications in younger and healthy adults. Therefore, many of us opposed the plans to vaccinate all such people. In our country 85 percent of people, or 115 crore people, being below the age of 50, would not have needed this vaccination. Even among the remaining 20 crore people above that age, if 40 percent had already been infected and recovered, vaccination would not have been necessary. Excluding these, only about 12 crore people above the age of 50, and those above 35 years of age with problems such as obesity, diabetes, heart disease etc. would have required the vaccination, if at all. We also filed a public interest petition in the state high court questioning the statement of the then higher education minister Ashwathanarayan that students who had not been vaccinated could not come to college from June 2021. But the government did not consider any of these, and most of the doctors also mocked and opposed.
There was no clear evidence that the new corona vaccines were safe. After the new coronavirus was identified in December 2019, the viral genome sequence was identified and published by Chinese scientists on January 12, 2020. Immediately thereafter the race for making a vaccine against it also began, and many types of vaccines were prepared using technologies that had not been used until then. According to the US CDC, it takes at least 10-15 years for a vaccine to be developed, tested in three phases, approved and put into use. But the new corona vaccines were developed in just six months, tested in just a couple of months, without much studies on safety and long-term side effects, and were given emergency use authorizations, all within one year.
The first vaccines from China and Russia arrived in July-August 2020. By the end of December 2020, the vaccine developed by Oxford University and AstraZeneca was approved by European countries, and vaccination soon started in countries like England and Denmark. Serum Institute of India, the largest vaccine manufacturer, obtained the licence from AstraZeneca to produce the same vaccine and offer it in India under the name of Covishield. India's own Bharat Biotech company developed its own vaccine (Covaxin) in collaboration with ICMR and went for approval. Our government, caught up in the vaccine race, hastily held meetings in early January 2021 and approved the emergency use of both of these vaccines even before reports of the studies done in our country were available.
When EUA was given to Covishield, reports of tests conducted in India hadn’t been published and the EUA was given on the basis of studies conducted in Britain, Brazil and Africa. For Covaxin, EUA was given in ‘clinical trial mode’, after only phase 2 trials, under the pretext of ‘the need for such a vaccine to control a British variant’, and the vaccine recipients were to be the subjects of its phase 3 trials!
The emergency use authorisation for these two vaccines was questioned and opposed by many experts, and the owners of those vaccine companies had a public spat. While Aadar Poonawala of Covishield pooh-poohed Covaxin, stating it was nothing more than water, Krishna Ella of Covaxin termed the studies on Covishield as poorly designed and stated that it had side effects in 60-70% of recipients! Senior vaccine scientist Dr. Gagandeep Kang declared her refusal to take either of these two vaccines, pointing out that reports of the trials conducted in India on Covishield hadn’t been published, nor any information was available on Covaxin. Senior virologist Dr. Jacob John declared that he won't take Covishield due to reported side effects. Senior immunologist Dr. Vineeta Bal termed it unethical and Hitlerian to start using vaccines without evidence of their ability to prevent infection. Senior virologist Dr. Shahid Jameel questioned the manner in which these vaccines were given authorisation. Dr. Soumya Swaminathan, chief scientist of the World Health Organization, said that universal use of vaccines couldn’t be allowed until the minimum standards of efficacy and safety were guaranteed. (I wrote all this in an article titled 'Is there an urgent need for a vaccine for a non-emergency corona infection?' published in Varthabharati on January 15, 2021). After a few months, most of them quietened down or changed their positions, even the fight between the vaccine makers went cold!
Let the Truth be known. If you read VB and like VB, please be a VB Supporter and Help us deliver the Truth to one and all.
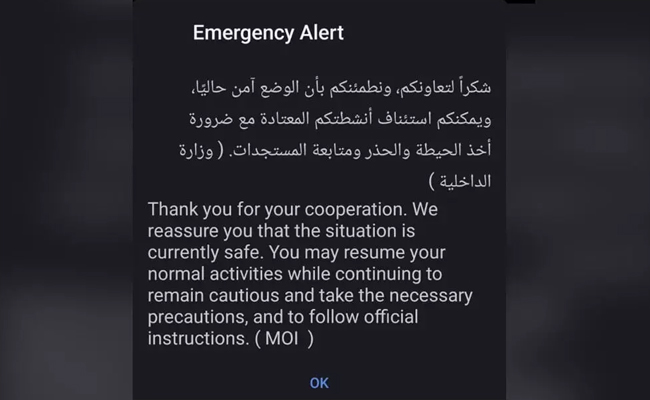
Dubai/Abu Dhabi: Residents and visitors across the United Arab Emirates received a fresh emergency alert on their mobile phones stating that the situation in the country is currently safe.
The message, issued by the Ministry of Interior (MOI), thanked people for their cooperation and reassured them that conditions were stable.
“Thank you for your cooperation. We reassure you that the situation is currently safe. You may resume your normal activities while continuing to remain cautious and take the necessary precautions, and to follow official instructions. (MOI),” the alert read.
The notification was sent in both Arabic and English through the country’s emergency alert system.
The advisory comes after earlier alerts warning of potential missile threats amid rising regional tensions. Authorities have urged the public to stay cautious and follow official guidance.