Ever since the vaccine maker Asta Zeneca’s admission before the UK court, the issue of severe adverse effects of corona vaccines has come to the fore again. The medical professionals who had brushed off the adverse effects as negligible and encouraged people to get vaccinated are now forced to justify their position. Were the side effects of these new vaccines indeed negligible?
Even when Covishield vaccine was given emergency use authorisation in India, there were early reports of possible side effects of the vaccine. In about a week after the vaccination was started on January 16, 2021 for doctors and health workers, there were reports of about 10 deaths among vaccine recipients, and some of us had raised the issue of safety of this vaccine and had questioned the rationale of using it without enough studies. In an interview to the periodical Mangalore Today in January 2021, I had pointed out these reports of at least ten deaths due to heart attacks and strokes after the vaccination and had called for investigations to know whether these deaths were related to the vaccine. By February-March 2021, there were reports of 5 deaths due to blood clotting and low platelet count (thrombotic thrombocytopenia) in Denmark, where 130,000 people (1 death in 26,000 recipients) had already received the same vaccine. Considering this, on March 11, 2021, Denmark and Norway stopped the use of this Oxford (Covishield) vaccine. Soon thereafter, Sweden and England, the parent countries of the makers of this vaccine, Astra-Zeneca and Oxford University, and also some other European countries as well as Australia either stopped the use or limited it to adults only. By the time Covishield vaccine was introduced to the general public in India in April 2021, all these reports were available, and we too had brought them to the attention of the government. When the Minister of Higher Education in Karnataka enforced corona vaccination as mandatory for entering colleges in Karnataka in May-June 2021, I had sent a legal notice to him stating that this vaccine had been withheld from being given to students and the young in other countries. This fact was also stated in the public interest petition filed in the state high court in June 2021. But all these warnings went unheeded and our students and youth were vaccinated. People were encouraged to get three doses without any need or basis. Reports of side effects in vaccine recipients were ignored and denied. Those who warned about these adverse reactions and unscientific methods were berated. Even though it was officially said that vaccination was optional, everyone was pressured to get vaccinated under the garbs of nationalism, sectarianism, and obedience of government orders etc. With all these, over 95 crore Indians were vaccinated.
Since the beginning of Corona vaccination, many reports of side effects have been published. A report of a multinational study on COVID 19 vaccines and adverse events of special interests was published on April 2, 2024. [Vaccine, 2 April 2024;42(9):2200] In Europe (Denmark, Finland, France, Scotland), Australia, New Zealand and Argentina, 21,97,37904 doses of mRNA vaccine and 2,30,93399 doses of Oxford (Covishield) vaccine were administered. Side effects observed within 42 days of administration were analyzed in this report. There was a statistically significant increase in Cerebral venous sinus thrombosis (OE ratio = 2.49) and Guillain-Barré Syndrome (OE ratio = 2.49) after a first dose of ChAdOx1 (Oxford) vaccine and myocarditis (OE ratio = 3.48) and acute disseminated encephalomyelitis (OE ratio = 3.78) following the first dose of mRNA-1273 vaccine. Notably, the study did not include data from India, where 175 crore doses of the Oxford vaccine were used, and it also lacked information on long-term side effects.
Vaccine induced thrombosis and thrombocytopenia (TTS) has been reported only with Oxford (Covishield) and Janssen vaccines. Studies from other countries estimated the incidence of TTS from 2 to 16 cases per million doses, which was higher among those who received the Oxford vaccine (npj Vaccines. 2022;7:141). What's more, these studies have reported that TTS occurs in 1 per million in those over 65 years of age, 3 per million in those aged 55-64 years, and 1 in 20,000 to 60,000, i.e. 17 to 50 per million, under 55 years of age. In other words, among those under the age of 55, infection fatality was 3 to 20 per million, while vaccine induced TTS was 17 to 50 per million; meaning that in people under 55, the vaccine was more riskier than the infection, and the infection was less riskier than the vaccine. In the age group of more than 60 years, infection fatality was 60 per million, vaccine induced TTS was one per million, making the vaccine safer than the infection. When it was known in Denmark in March 2021 that one in 26000 had died from the Oxford vaccine and that the vaccine was immediately withdrawn, why was there a need to give that vaccine to everyone in our country?
In India, comprehensive and accurate data about adverse events following immunization (AEFI) are not available and it is not even clear whether such information has been recorded honestly. If TTS has occurred in India at the same rate as elsewhere in the world, about 22,000 cases of TTS and related deaths might have occurred here. According to Awaken India Movement, which has actively collected and published information about AEFI, there have been 19273 deaths recorded in connection with COVID vaccination, but authenticity of this data cannot be verified. According to the official information provided by the government in Parliament, Supreme Court of India and under the Right to Information Act, out of a total of 219.6 crore doses of vaccines administered in our country, 92114 (0.0042%) cases of AEFI were recorded, of which 2782 were serious, and 1148 deaths occurred, 90 per cent of the doses and 92 per cent of the deaths were of Covishield. In Canada, Brazil and Argentina, AEFI were recorded in 0.06 per cent of vaccinees, whereas in India it was 10-15 times lower, suggesting that AEFI were possibly not recorded properly here.
Cases of blood clotting due to Covishield have not only involved the cerebral veins, but also there have been reports of blood clots in the veins and arteries of the intestines, retina, limbs, etc., and neurological complications such as Guillain Barre syndrome and cardiac complications such as myocarditis have also been reported. As the vaccines were given emergency use authorization on the basis of hasty and short-term trails of only a few weeks, without any studies on long term adverse effects, neither the doctors nor the general public had enough information about the possible adverse effects. Therefore, even when such adverse events did occur following vaccinations, they were not attributed to vaccines, or were deliberately rejected as unrelated to vaccines, and were not recorded as AEFI. For this reason, it cannot be ruled out that the actual number of people who suffered AEFI due to Covishield and other vaccines may be many times higher than what has been recorded.
As early as March-April 2021, scientists in Denmark had identified that some special antibody produced against the Oxford (Covishield) vaccine may be responsible for TTS. Both Oxford (Covishield) and Janssen vaccines are recombinant vaccines, using adeno viruses (of chimpanzees and humans) as vectors for the corona virus spike protein gene, and have been shown by many studies to produce antibodies against platelets (anti-platelet factor 4 antibody), resulting in thrombosis and thrombocytopenia. A recent study conducted at Flinders University in Australia has also reconfirmed these findings. Some studies have shown that although these antibodies disappear within 40-50 days in most people (about 85%), some may persist even after six months, and in 3-9% the risk of thrombosis may persist for a long time (Journal of Clinical Medicine. 2024;13(4):1012) Whether the increasing cases of sudden cardiac arrest, heart attack and stroke are related to TSS and vaccine induced antibodies cannot be answered without further studies.
For Covaxin, which was given emergency approval without phase III trials, ‘under clinical trial mode’, neither the final report of phase III trials nor of AEFIs have been published so far. A few days ago, experts from Banaras Hindu University published a report that 70% of those who received Covaxin experienced adverse effects. The ICMR warned the Banaras University that the study was flawed and sought removal of its name from acknowledgments. Questions to ICMR and Bharat Biotech to show a better study has remained unanswered so far!
It is therefore time now for our government, doctors, medical organizations and vaccine manufacturing companies to give honest, evidence-based answers to our 95 crore people who were inadequately informed and pressured to get vaccinated unnecessarily. After the Astra Zeneca company admitted the adverse effects in court, a doctor from Karnataka, speaking to the media, compared the corona vaccine to a bus-train journey, and stated that just as people travel in buses-trains-airplanes even after being aware of the risks of accidents, vaccinations too can be associated with risks and these risks need to be taken, considering the benefits of the vaccines. But the fact is that for the real bus or train alluded to by this doctor, passengers buy the tickets on their own volition, and board them on their own after knowing fully well the risks of accidents. The covid vaccine bus, on the other, was touted as very safe, and without informing anyone much about any risks involved, it was as if the government and these doctors pushed our people into it for free travel, and now, after 2 years of travel, are scaring the passengers, travelling forcibly, that it can meet with accident leading to serious injuries. Doctors who forced our people to get vaccinated, and are now struggling to justify it, need to know the difference between these two buses.
Let the Truth be known. If you read VB and like VB, please be a VB Supporter and Help us deliver the Truth to one and all.
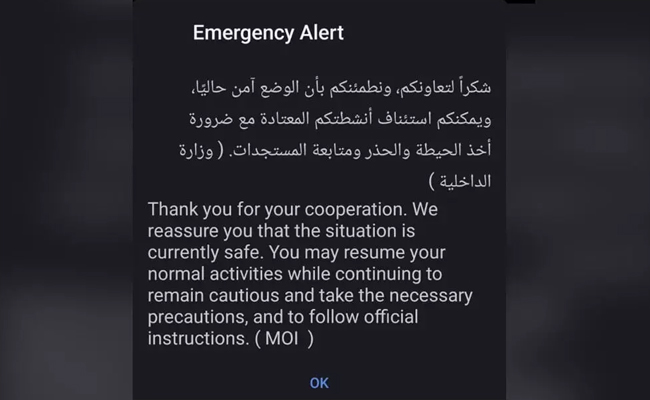
Dubai/Abu Dhabi: Residents and visitors across the United Arab Emirates received a fresh emergency alert on their mobile phones stating that the situation in the country is currently safe.
The message, issued by the Ministry of Interior (MOI), thanked people for their cooperation and reassured them that conditions were stable.
“Thank you for your cooperation. We reassure you that the situation is currently safe. You may resume your normal activities while continuing to remain cautious and take the necessary precautions, and to follow official instructions. (MOI),” the alert read.
The notification was sent in both Arabic and English through the country’s emergency alert system.
The advisory comes after earlier alerts warning of potential missile threats amid rising regional tensions. Authorities have urged the public to stay cautious and follow official guidance.