New Delhi: RTI activist Saket Gokhale on Wednesday made shocking claims through a Facebook post saying there was a mystery in vaccine trials of Bharat Biotech’s Phase 1 and 2.
According to Gokhale, Bharat Biotech registered its Phase 1 and 2 trials for Covaxin with India’s Clinical Trials Registry (CTR) – a division of ICMR – which happens to be Bharat Biotech’s partner. He claimed that Bharat Biotech registered Phase 1 details on July 1, 2020.
“Remember - this was the time when ICMR issued a shocking letter saying "vaccine must be ready by Independence Day" so that PM Modi could earn brownie point,” he wrote in the post.
He also shared pictures which added that Phase 1 registered on July 1, 2020, was to take eight months i.e. by March 2021 but details of Phase 2 were registered on September 8, 2020, even before Phase 1 was completed.
“This was literally within 2 months of registering Phase 1 trial” he added.
“Now let's look at the duration of Phase 1 & Phase 2 trials as declared by Bharat Biotech themselves. Phase 1 (registered on 1 Jul 2020) was to take 8 months i.e. by March 2021. Phase 2 (registered on 8 Sep 2020) was to take 1 year & 3 months i.e. by December 2021. Phase 3 of Covaxin trials were registered on 9th November 2020 with a sample size of 25,800 participants. Registered duration for Phase 3 is 1 year i.e. by Nov 2021” he wrote in the post.
“Basically, Bharat Biotech registered Phase 2 & Phase 3 trials before Phase 1 was not even completed,” he said.
“To sum it up: Phase 1 to be completed by Mar 2021, Phase 2 to be completed by Dec 2021, Phase 3 to be completed by Nov 2021 (that's, bizarrely, 1 month BEFORE Phase 2). And yet - Bharat Biotech's Covaxin has been approved by the Indian govt in January 2021” he added.
“On what basis was this approval given when, by Bharat Biotech's own registration documents, Phase 1 completion is still 3 months away? Is this why the Modi govt is reluctant to share data for Bharat Biotech's Phase 1 & 2 trials publicly? The TOTAL number of volunteers in Bharat Biotech's Phase 1 AND 2 trials is 1249 people. For a country with a population of 1.3 billion people. How on earth is this vaccine "110% safe" & "works against the mutated strain"? Where's the Phase 1 & 2 data to prove this?” Gokhale questioned.
Gokhale demanded that the Modi government must immediately release the Phase 1 and Phase 2 data of Bharat Biotech’s Covaxin trials to the public. “It must also explain how trials, which were supposed to take 1.5 years, got done in 5 months. The reluctance to share any data is a massive red flag” he further wrote.
Let the Truth be known. If you read VB and like VB, please be a VB Supporter and Help us deliver the Truth to one and all.
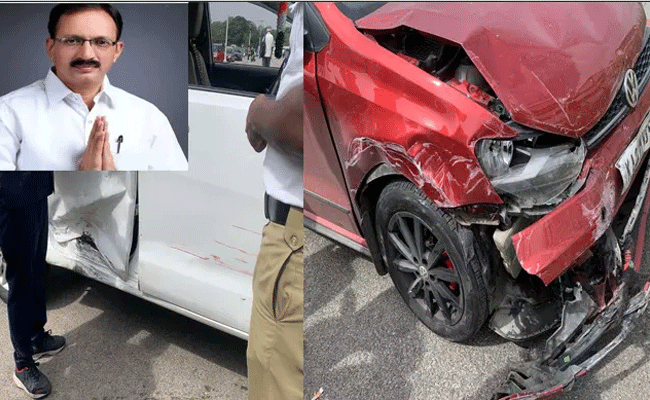
Bengaluru: In a recent incident near the Vidhana Soudha, Bailahongala MLA Mahantesh Kaujalagi was involved in a car accident. The mishap occurred as the MLA was departing from the MLA's House and was struck by a polo vehicle while exiting the Vidhana Soudha premises.
Fortunately, the MLA sustained only minor injuries in the collision. Following the accident, Kaujalagi was immediately transported to the hospital in another vehicle for medical attention.
The incident happened within the jurisdiction of the Cubbon Park Traffic Police Station.